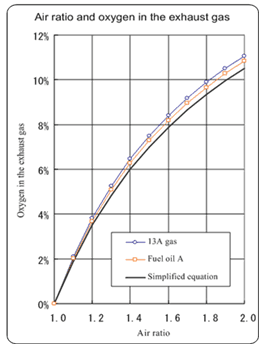
- The simplified equation for obtaining the air ratio is: Air ratio = 21/(21 – O2% value) where O2 is the oxygen content obtained by analysis (dry base value).
- Normally, the air ratio may be calculated by this equation. The criteria of the Law Concerning Rational Use of Energy specify this equation.
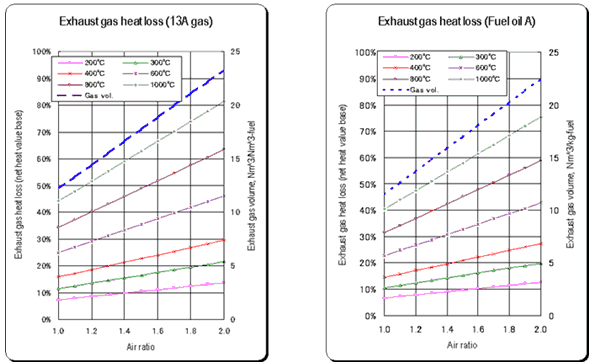
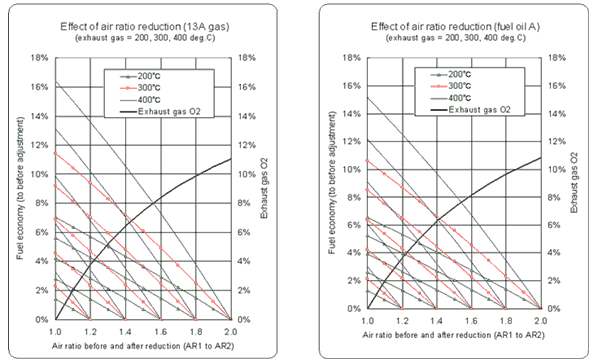
- As the air ratio becomes large, the difference between the result obtained by the simplified equation and the real value becomes large. (See the graph.)
- The air ratio is defined as "real air value/theoretical air volume.
- " The air ratio of 1 (one) represents an ideal state in which no more than required air is used for combustion (theoretical combustion).
- The air in excess of air ratio at 1 (one) is called gexcess airh which causes heat loss by carrying out air from the stack at heated state.
- The air ratio short of 1 (one) causes incomplete combustion, associated with the incomplete combustion heat loss.
|
 |
|